A Review of Electronic Skin and Its Application in Clinical Diagnosis and Treatment of Traditional Chinese Medicine
-
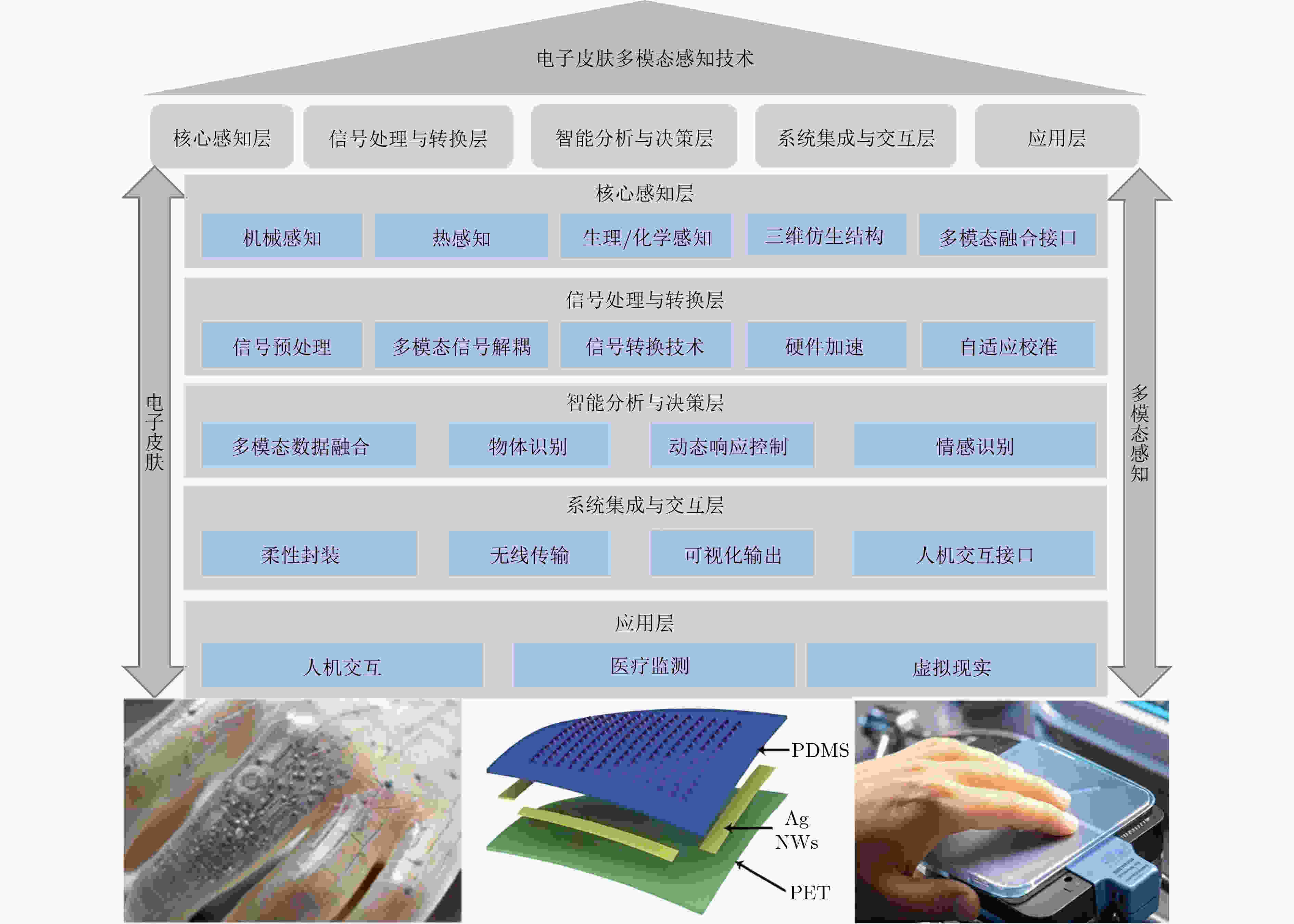
摘要: 随着柔性电子技术与智能传感技术的突破,电子皮肤为中医诊疗客观化、精准化提供了创新解决方案。该文系统综述了中医诊疗对柔性电子皮肤的技术需求,从材料创新、多模态感知融合及自供电技术突破等维度,剖析柔性电子皮肤在中医诊疗中的技术演进路径与前沿发展趋势;最终,通过电子皮肤在中医诊疗中疾病诊断的可行性评估与技术挑战分析,提出适配中医诊疗场景的柔性电子皮肤技术路线,为构建数据驱动的新型中医诊疗范式提供理论依据与创新策略。电子皮肤为中医临床智能化诊疗提供了创新思路。电子皮肤在中医领域的应用极具挑战性,未来需突破材料、多模态数据融合与中医知识图谱等方面多重壁垒,构建中医现代化发展创新思路。Abstract:
Integrating Electronic Skin (e-skin) into Traditional Chinese Medicine (TCM) diagnostics offers a novel approach to addressing long-standing issues of standardization and objectivity. Core diagnostic practices in TCM-pulse assessment, tongue analysis, and acupuncture, are predominantly based on subjective interpretation, which hinders reproducibility and limits broader clinical acceptance. This review examines recent advances in e-skin technology, including flexible electronics, multimodal sensing, and Artificial Intelligence (AI), and discusses their potential to support quantifiable, data-driven diagnostic frameworks. These developments may provide a technological basis for modernizing TCM while maintaining its holistic orientation. This review systematically examines the convergence of TCM clinical requirements and e-skin technologies through a comprehensive survey of over 60 peer-reviewed studies and patents published between 2015 and 2024. First, the current state of e-skin research is mapped onto the diagnostic needs of TCM, with a focus on material flexibility, multisensory integration, and energy autonomy. Second, key technical challenges are identified through comparative analysis of sensor performance metrics (e.g., sensitivity, durability) and TCM-specific biomarker detection requirements. Third, a framework is proposed for optimizing e-skin architectures in accordance with TCM’s systemic diagnostic logic. The analysis highlights three technical domains: (1) Material innovations: Graphene-polymer composites and liquid metal-hydrogel interfaces that enable conformal adherence to dynamic biological surfaces ( Fig. 3 ). (2) Multimodal sensing: Heterogeneous sensor arrays capable of synchronously capturing pulse waveforms, tongue coatings, and acupoint bioimpedance (Table 1 ). (3) AI-driven signal interpretation: Deep learning models such as ResNet-1D and transformer networks for classifying TCM pulse patterns and body constitutions.e-skin technologies have advanced significantly in supporting the digital transformation of TCM through innovations in materials, sensing functions, and algorithmic design. In pulse diagnosis, graphene-based sensor arrays achieve 89.3% classification accuracy across 27 pulse categories ( Table 2 ), exceeding manual assessments (Kappa: 0.72 vs. 0.51) by quantifying nuanced differences in pulse types such as “slippery” and “wiry” (Fig. 1 ). For tongue diagnosis, MXene-enabled multispectral imaging (400~1000 nm) supports automated analysis of coating thickness with an F1-score of 0.91, and reveals thermal-humidity gradients correlated with Yang Deficiency patterns (Fig. 6 ). Acupuncture standardization has improved through the use of piezoresistive needle arrays, which reduce insertion depth errors to ±0.3 mm. Integration with machine learning further enables classification of nine TCM body constitutions at 86.4% accuracy, supporting personalized therapeutic strategies (Fig. 5 ). Despite these achievements, key technical limitations remain. Material degradation and signal synchronization latency over 72 ms restrict real-time applications. Variability in sensor specifications (sampling rates from 50 to 2,000 Hz) and the lack of quantifiable biomarkers for TCM concepts such as Qi-Stagnation continue to hinder clinical validation (Table 2 ). Future research should focus on: (1) Self-healing materials: Bioinspired hydrogels with strain tolerance over 300% and enhanced fatigue resistance. (2) Edge-AI architectures: Lightweight transformer-CNN hybrids optimized for reduced latency (<20 ms). (3) TCM-specific biomarkers: Electrochemical sensors designed to detect molecular correlates of Yin-Yang imbalances.This review outlines a roadmap for modernizing TCM through e-skin integration by aligning technological advances with clinical requirements. Three key insights are emphasized: (1) Material-device co-design: Engineering stretchable electronics to accommodate the dynamic diagnostic contexts of TCM. (2) Multimodal data fusion: Combining pulse, tongue, and meridian signals to support systemic pattern differentiation. (3) Regulatory frameworks: Establishing TCM-oriented standards for sensor validation and clinical reliability. Emerging applications-including Internet of Things (IoT)-connected e-skin patches for continuous Zang-Fu organ monitoring and AI-guided acupuncture robotics-illustrate the field’s transformative potential. By 2030, the interdisciplinary integration of flexible electronics, artificial intelligence, and TCM principles is projected to enable e-skin diagnostic systems to be adopted in 40% of tertiary hospitals, supporting the transition of TCM toward a globally recognized precision medicine paradigm. -
表 1 不同传感方式在中医诊疗中的优劣势分析对比表
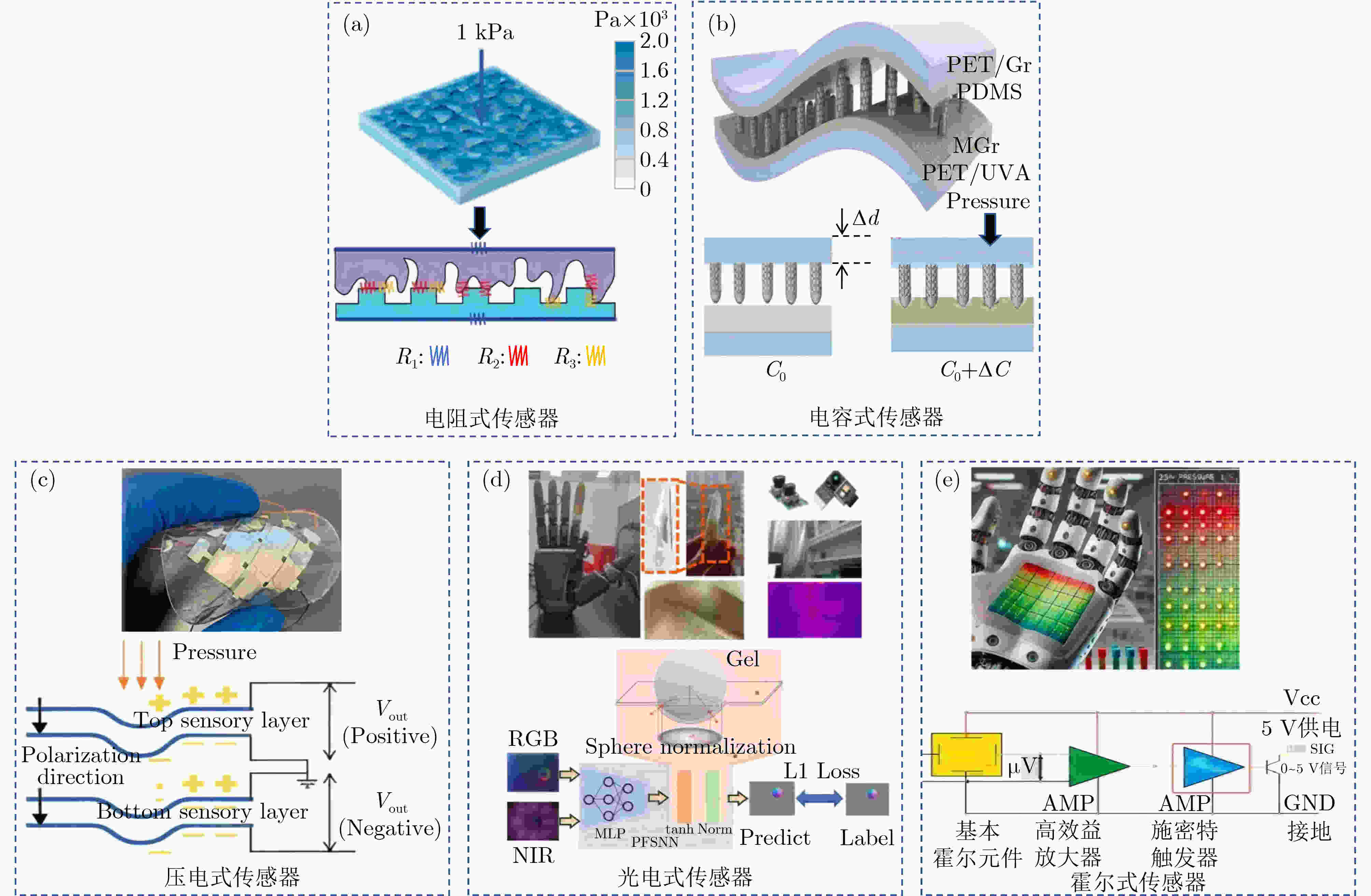
传感类型 适用场景 关键指标 中医诊疗优势 主要挑战 电阻式 脉诊压力分布 灵敏度、抗干扰性 高密度脉象采集 稳定性差 电容式 穴位微形变 超高分辨率(1μm) 动态组织反馈 湿度敏感 压电式 动态脉象与针灸 能量回收效率 自供电与实时力反馈 缺乏静态信号 光电式 舌诊与皮下监测 空间分辨率高 非接触式高精度成像 集成难度高 霍尔式 推拿力方向追踪 角度误差 抗干扰性强 适配场景有限 复合式 电阻+电容:脉诊与慢病管理 兼顾压力灵敏度与微形变监测 根据需求融合多模态信息,
实现多传感信号观测抗干扰性、融合性、
稳定性压电+光电:针灸与推拿 力学反馈与组织状态同步监测 光电+电阻:舌诊与体质辨识 多光谱成像与体表湿度关联等 表 2 中医临床诊疗对电子皮肤的技术需求映射
传感器要求项目 指标等要求 标准依据 是否成熟 高灵敏度与多模态感知 亚毫牛级压力分辨率(如0.1~10 kPa范围)和高频动态响应能力(>100 Hz);需集成
压阻(压力)、电容(微形变)、温度、湿度、电化学(生物电)等多模态传感器,
同时采集脉象压力、穴位电导率、体表温度中医“四诊合参” 否 柔性贴合与生物相容性 超薄(<1 mm)、可拉伸(>50%形变)、低模量(接近皮肤)特性 ISO 10993 生物
相容性认证否 动态环境下的抗干扰能力 消除肢体运动、呼吸震动等噪声;适应温度(10~40 °C)、湿度(30%~90% RH)波动,
传感器输出需具备温度自补偿功能无 否 中医特征信号解析能力
开发针对脉象“位、数、形、势”特征的专用算法,“气血虚实”“寒热表里”等抽象概念的量化表达无 否 长期稳定性与低功耗设计 数周至数月,低功耗传感设计,能量收集技术 否 标准化与临床适配性 《中医脉象采集设备技术规范》等 尚不完整 否 -
[1] LIU Zhi, HU Xiaonan, BO Renheng, et al. A three-dimensionally architected electronic skin mimicking human mechanosensation[J]. Science, 2024, 384(6699): 987–994. doi: 10.1126/science.adk5556. [2] HAMMOCK M L, CHORTOS A, TEE B C K, et al. 25th anniversary article: The evolution of electronic skin (E‐Skin): A brief history, design considerations, and recent progress[J]. Advanced Materials, 2013, 25(42): 5997–6038. doi: 10.1002/adma.201302240. [3] 任秦博, 王景平, 杨立, 等. 用于电阻式柔性应变传感器的导电聚合物复合材料研究进展[J]. 材料导报, 2020, 34(1): 80–94. doi: 10.11896/cldb.19100229.REN Qinbo, WANG Jingping, YANG Li, et al. Research progress of conductive polymer composites for resistive flexible strain sensors[J]. Materials Review, 2020, 34(1): 80–94. doi: 10.11896/cldb.19100229. [4] 张嘉琰, 温良恭, 张立平, 等. 近5年可穿戴技术在中医方面的应用[J]. 世界中医药, 2022, 17(16): 2358–2365. doi: 10.3969/j.issn.1673-7202.2022.16.023.ZHANG Jiayan, WEN Lianggong, ZHANG Liping, et al. Application of wearable technology in traditional Chinese medicine in the recent five years[J]. World Chinese Medicine, 2022, 17(16): 2358–2365. doi: 10.3969/j.issn.1673-7202.2022.16.023. [5] https://www.gov.cn/gongbao/content/2022/content_5686029.htm. [6] https://www.most.gov.cn/xxgk/xinxifenlei/fdzdgknr/fgzc/gfxwj/gfxwj2022/202301/t20230116_184238.html. [7] CAO Yujie, LI Ping, ZHU Yirun, et al. Artificial intelligence-enabled novel atrial fibrillation diagnosis system using 3D pulse perception flexible pressure sensor array[J]. ACS Sensors, 2025, 10(1): 272–282. doi: 10.1021/acssensors.4c02395. [8] CHU Yuwen, LUO C H, CHUNG Y F, et al. Using an array sensor to determine differences in pulse diagnosis-Three positions and nine indicators[J]. European Journal of Integrative Medicine, 2014, 6(5): 516–523. doi: 10.1016/j.eujim.2014.04.003. [9] SALAMA M M A and BARTNIKAS R. Determination of neural-network topology for partial discharge pulse pattern recognition[J]. IEEE Transactions on Neural Networks, 2002, 13(2): 446–456. doi: 10.1109/72.991430. [10] COHEN N L, PAULSEN R E, and WHITE M H. Clinical observation on the treatment of 112 cases of hypertensive disease with syndrome differentiation and classification[J]. Guiding Journal of TCM, 2006, 288(6): C1342–C1356. doi: 10.1152/ajpcell.00315.2004. [11] LIU Zhi, ZHANG D, YAN Jingqi, et al. Classification of hyperspectral medical tongue images for tongue diagnosis[J]. Computerized Medical Imaging and Graphics, 2007, 31(8): 672–678. doi: 10.1016/j.compmedimag.2007.07.008. [12] LEE T C, LO L C, and WU Fangchen. Traditional Chinese medicine for metabolic syndrome via TCM pattern differentiation: Tongue diagnosis for predictor[J]. Evidence-Based Complementray and Alternative Medicine, 2016, 2016: 1971295. doi: 10.1155/2016/1971295. [13] ZHAO Mei, ZHOU Hengyu, WANG Jing, et al. A new method for identification of traditional Chinese medicine constitution based on tongue features with machine learning[J]. Technology and Health Care, 2024, 32(5): 3393–3408. doi: 10.3233/THC-240128. [14] KIM M S, SHIN H J, and PARK Y K. Design concept of high-performance flexible tactile sensors with a robust structure[J]. International Journal of Precision Engineering and Manufacturing, 2012, 13(11): 1941–1947. doi: 10.1007/s12541-012-0256-3. [15] YU Tong, LI Jinghua, YU Qi, et al. Knowledge graph for TCM health preservation: Design, construction, and applications[J]. Artificial Intelligence in Medicine, 2017, 77: 48–52. doi: 10.1016/j.artmed.2017.04.001. [16] 赵庭煜, 邵亮, 姬占有, 等. 高灵敏、强粘附性导电水凝胶的制备及在柔性传感中的应用[J]. 材料导报, 2025, 39(4): 208–218. doi: 10.11896/cldb.24010212.ZHAO Tingyu, SHAO Liang, JI Zhanyou, et al. Preparation of a highly sensitive, strongly adhesive conductive hydrogel and its application in flexible sensing[J]. Materials Review, 2025, 39(4): 208–218. doi: 10.11896/cldb.24010212. [17] YAO Hongbin, GE Jin, WANG Changfeng, et al. Pressure sensors: A flexible and highly pressure-sensitive graphene-polyurethane sponge based on fractured microstructure design (Adv. Mater. 46/2013)[J]. Advanced Materials, 2013, 25(46): 6691. doi: 10.1002/adma.201370292. [18] WANG Gaofeng, MENG Lingxian, JI Xinyi, et al. Nacre-inspired MXene-based film for highly sensitive piezoresistive sensing over a broad sensing range[J]. Bio-Design and Manufacturing, 2024, 7(4): 463–475. doi: 10.1007/s42242-024-00292-4. [19] MENG Lei, SHAO Changyou, CUI Chen, et al. Autonomous self-healing silk fibroin injectable hydrogels formed via surfactant-free hydrophobic association[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 1628–1639. doi: 10.1021/acsami.9b19415. [20] WEI Jingjiang, CHEN H, PAN Fei, et al. 3D-printable liquid metal-based hydrogel for use as a multifunctional epidermal sensor[J]. Nanoscale, 2025, 17(10): 5681–5688. doi: 10.1039/D4NR04997G. [21] ADJI A, HIRATA K, HOEGLER S, et al. Noninvasive pulse waveform analysis in clinical trials: Similarity of two methods for calculating aortic systolic pressure[J]. American Journal of Hypertension, 2007, 20(8): 917–922. doi: 10.1016/j.amjhyper.2007.03.006. [22] ZHANG Fang, SUN Gaoqi, ZHAO Rong, et al. Zwitterion-modified MXene quantum dot as a nanocarrier for traditional Chinese medicine sanguinarine delivery and its application for photothermal-chemotherapy synergistic antibacterial and wound healing[J]. Langmuir, 2024, 40(22): 11381–11389. doi: 10.1021/acs.langmuir.3c03992. [23] NAKAGUCHI T, TAKEDA K, ISHIKAWA Y, et al. Proposal for a new noncontact method for measuring tongue moisture to assist in tongue diagnosis and development of the tongue image analyzing system, which can separately record the gloss components of the tongue[J]. Biomed Research International, 2015, 2015(1): 249609. doi: 10.1155/2015/249609. [24] LI Sen, HUANG Jiantao, WANG Meilan, et al. Structural electronic skin for conformal tactile sensing (Adv. Sci. 33/2023)[J]. Advanced Science, 2023, 10(33): 2370227. doi: 10.1002/advs.202370227. [25] YANG Minjin, CHUNG H, KIM Y, et al. A body-scale robotic skin using distributed multimodal sensing modules: Design, evaluation, and application[J]. IEEE Transactions on Robotics, 2025, 41: 96–109. doi: 10.1109/TRO.2024.3502204. [26] SUN Chanming, LIU Huifang, WANG Jiaqi, et al. Flexible piezoelectric sensor based on PVDF/ZnO/MWCNT composites for human motion monitoring[J]. Organic Electronics, 2025, 144: 107290. doi: 10.1016/j.orgel.2025.107290. [27] XUE Haoyue, JIN Jing, TAN Zhi, et al. Flexible, biodegradable ultrasonic wireless electrotherapy device based on highly self-aligned piezoelectric biofilms[J]. Science Advances, 2024, 10(22): eadn0260. doi: 10.1126/sciadv.adn0260. [28] LIU Wanling, YE Juncheng, WANG Yanlang, et al. Multimodal antibacterial E-skin patch driven by oxidative stress for real-time wound-status monitoring and integrated treatment of chronic wounds[J]. Advanced Functional Materials, 2025, 35(22): 2424698. doi: 10.1002/adfm.202424698. [29] NIU Li, PENG Xiao, CHEN Lijun, et al. Industrial production of bionic scales knitting fabric-based triboelectric nanogenerator for outdoor rescue and human protection[J]. Nano Energy, 2022, 97: 107168. doi: 10.1016/j.nanoen.2022.107168. [30] YANG Peng, LIU Zhaoqi, QIN Siyao, et al. A wearable triboelectric impedance tomography system for noninvasive and dynamic imaging of biological tissues[J]. Science Advances, 2024, 10(51): eadr9139. doi: 10.1126/sciadv.adr9139. [31] ZI Yunlong, LIN Long, WANG Jie, et al. Triboelectric-pyroelectric-piezoelectric hybrid cell for high-efficiency energy-harvesting and self-powered sensing[J]. Advanced Materials, 2015, 27(14): 2340–2347. doi: 10.1002/adma.201500121. [32] OKODUWA S I R, IGIRI B E, TAGANG J I, et al. Therapeutic smart-footwear approach for management of neuropathic diabetic foot ulcers: Current challenges and focus for future perspective[J]. Medicine in Novel Technology and Devices, 2024, 23: 100311. doi: 10.1016/j.medntd.2024.100311. [33] YANG Li, CHEN Xue, DUTTA A, et al. Thermoelectric porous laser-induced graphene-based strain-temperature decoupling and self-powered sensing[J]. Nature Communications, 2025, 16(1): 792. doi: 10.1038/s41467-024-55790-x. [34] LEE K P, WANG Zhijun, ZHENG Lin, et al. Enhancing orthotic treatment for scoliosis: Development of body pressure mapping knitwear with integrated FBG sensors[J]. Sensors, 2025, 25(5): 1284. doi: 10.3390/s25051284. [35] WANG Wenhui, WANG Sheng, LI Zimu, et al. A flexible protective electronic skin with tunable anti-impact and thermal insulation properties for potential rescue applications[J]. Journal of Materials Chemistry C, 2025, 13(3): 1146–1156. doi: 10.1039/D4TC04519J. [36] CHEN Zhongda, SONG Jun, LU Yu, et al. Mechanical compatibility in stitch configuration and sensor adhesion for high-fidelity pulse wave monitoring[J]. Advanced Science, 2025, 12(14): 2415608. doi: 10.1002/advs.202415608. [37] ZHANG Heyi, WANG Xin, MENG Zhaopeng, et al. Integrating large language models with knowledge graphs in traditional Chinese medicine consultation: A case study[C]. International Joint Conference on China Conference on Knowledge Graph and Semantic Computing and International Joint Conference on Knowledge Graphs, Chongqing, China, 2025: 360–366. doi: 10.1007/978-981-96-1809-5_28. [38] TIAN Yuqi, YANG Kai, WANG Yicong, et al. Self-adaptive epidermal blood flow sensor for high-flux vascular access monitoring of hemodialysis patients[J]. NPJ Flexible Electronics, 2024, 8(1): 62. doi: 10.1038/s41528-024-00342-y. [39] CHENG Rong, LIU Zixuan, LI Meng, et al. Peripheral nerve regeneration with 3D printed bionic double-network conductive scaffold based on GelMA/chitosan/polypyrrole[J]. International Journal of Biological Macromolecules, 2025, 304: 140746. doi: 10.1016/j.ijbiomac.2025.140746. [40] XU Chen, WANG Yiran, ZHANG Jingyan, et al. Three-dimensional micro strain gauges as flexible, modular tactile sensors for versatile integration with micro- and macroelectronics[J]. Science Advances, 2024, 10(34): eadp6094. doi: 10.1126/sciadv.adp6094. [41] ZHANG Xingya, DAI Hongfei, JI Mengnan, et al. A flexible piezoresistive strain sensor based on AgNWs/MXene/PDMS sponge[J]. Journal of Materials Science: Materials in Electronics, 2025, 36(8): 452. doi: 10.1007/s10854-025-14494-8. [42] ZHANG Tianxue, LUO Yimin, NI Yuting, et al. Research on vascular thrombosis detection methods based on fiber Bragg grating sensing[J]. IEEE Sensors Journal, 2025, 25(1): 489–497. doi: 10.1109/jsen.2024.3471824. [43] ZHANG Shixin, YANG Yiyong, SUN Yuhao, et al. Artificial skin based on Visuo-tactile sensing for 3D shape reconstruction: Material, method, and evaluation[J]. Advanced Functional Materials, 2025, 35(1): 2411686. doi: 10.1002/adfm.202411686. [44] KHALIL S F, MOHKTAR M S, and IBRAHIM F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases[J]. Sensors, 2014, 14(6): 10895–10928. doi: 10.3390/s140610895. [45] CHEN Ming, DING Zhi, WANG Weidong, et al. High-sensitivity flexible strain sensor with the inverted pyramid microstructure array based on stress-induced regular linear cracks[J]. Journal of Electronic Materials, 2025, 54(1): 241–250. doi: 10.1007/s11664-024-11474-2. [46] NARESH M, RAMESH M, and JADHAV A B. Optimized SVM-based model for health monitoring of joints in a multi-story 3D steel frame structure[J]. Asian Journal of Civil Engineering, 2025, 26(4): 1837–1846. doi: 10.1007/s42107-025-01293-z. [47] YANG Ping’an, ZHAO Jingyuan, LI Rui, et al. Construction of laser-induced graphene/silver nanowire composite structures for low-strain, high-sensitivity flexible wearable strain sensors[J]. Science China Technological Sciences, 2024, 67(11): 3524–3534. doi: 10.1007/s11431-024-2789-4. [48] GAO Fupeng, LIU Chunxiu, ZHANG Lichao, et al. Wearable and flexible electrochemical sensors for sweat analysis: A review[J]. Microsystems & Nanoengineering, 2023, 9: 1. doi: 10.1038/s41378-022-00443-6. [49] 吴恙, 张竹绿, 于彤, 等. 基于多模态的智能中医体质推荐系统构建研究[J]. 中国数字医学, 2025, 20(6): 85–91. doi: 10.3969/j.issn.1673-7571.2025.06.014.WU Yang, ZHANG Zhulü, YU Tong, et al. Research on the construction of intelligent TCM constitution recommendation system based on multimodality[J]. China Digital Medicine, 2025, 20(6): 85–91. doi: 10.3969/j.issn.1673-7571.2025.06.014. [50] GU Tianyu, YAN Zhuangzhi, and JIANG Jiehui. Classifying Chinese medicine constitution using multimodal deep-learning model[J]. Chinese Journal of Integrative Medicine, 2024, 30(2): 163–170. doi: 10.1007/s11655-022-3541-8. [51] CHOI M, YANG Dongmin, and LEE K W. A development of a digital tongue diagnosis system using the tongue color analysis of the each taste region[J]. Journal of the Korea Institute of Information and Communication Engineering, 2015, 19(2): 428–434. doi: 10.6109/jkiice.2015.19.2.428. [52] HU Hailong, LI Yaqian, ZHENG Zeyu, et al. A traditional Chinese medicine prescription recommendation model based on contrastive pre-training and hierarchical structure network[J]. Expert Systems with Applications, 2025, 268: 126318. doi: 10.1016/j.eswa.2024.126318. [53] LEGNER C, KALWA U, PATEL V, et al. Sweat sensing in the smart wearables era: Towards integrative, multifunctional and body-compliant perspiration analysis[J]. Sensors and Actuators A: Physical, 2019, 296: 200–221. doi: 10.1016/j.sna.2019.07.020. [54] HONG Tianzeng, XUE Jie, LI Haonan, et al. Vertical graphene/carbon nanotube/ polydimethylsiloxane composite films for multifunctional stretchable strain sensors[J]. Chemical Engineering Journal, 2025, 519: 164747. doi: 10.1016/j.cej.2025.164747. [55] 陈可居, 钟利, 张云, 等. 基于多视图舌象特征融合的中医证型辨识[J]. 计算机应用研究, 2025, 42(7): 2116–2122. doi: 10.19734/j.issn.1001-3695.2024.11.0471.CHEN Keju, ZHONG Li, ZHANG Yun, et al. Traditional Chinese medicine syndrome identification based on multi-view tongue image feature fusion[J]. Application Research of Computers, 2025, 42(7): 2116–2122. doi: 10.19734/j.issn.1001-3695.2024.11.0471. [56] BANDODKAR A J, JEERAPAN I, and WANG J. Wearable chemical sensors: Present challenges and future prospects[J]. ACS Sensors, 2016, 1(5): 464–482. doi: 10.1021/acssensors.6b00250. [57] YUAN Siqing, FAN Zebin, WANG Guangji, et al. Fabrication of flexible and transparent metal mesh electrodes using surface energy-directed assembly process for touch screen panels and heaters[J]. Advanced Science, 2023, 10(34): 2304990. doi: 10.1002/advs.202304990. [58] COOPER C B, ROOT S E, MICHALEK L, et al. Autonomous alignment and healing in multilayer soft electronics using immiscible dynamic polymers[J]. Science, 2023, 380(6648): 935–941. doi: 10.1126/science.adh0619. -




 下载:
下载:

 下载:
下载:
